Clinical Trials That Include Genomic Biomarkers Should Pave the Way for Precision Medicine

The healthcare landscape is shifting from ‘one-drug-treats-all’ to a paradigm focused on personalized medicine. Increasingly, clinicians are tailoring treatments to a patient’s specific genetic mutations. While the number of precision therapies targeting these mutations has grown, only recently have costs dropped to a low enough level that physicians can sequence an individual’s genomic profile and identify his or her mutations. Empowered by the cost declines of next generation DNA sequencing (NGS), diagnostic providers such as Veracyte (VCYT), Exact Sciences (EXAS), and Guardant Health (GH) are advancing personalized medicine by matching patients to precision therapies. ARK believes that therapeutics companies increasingly will use NGS in clinical trials, creating targeted therapies that ultimately will supplant traditional chemotherapies.
On average, humans inherit five million small genetic mutations called single nucleotide polymorphisms (SNPs). While most SNPs are benign, others can disrupt important biological processes. The gene TP53, for example, encodes a protein that first surveys the body for damaged cells that might trigger cancer and then decides whether to repair or destroy them. NGS can surface harmful SNPs in a patient’s TP53 gene, giving oncologists the opportunity to choose more effective therapeutic strategies. TP53 is just one of many genomic ‘biomarkers’, or genetic targets that call for precision therapies. Oncology accounts for the majority of genomic biomarkers tied to FDA-approved targeted therapies today, as shown below.
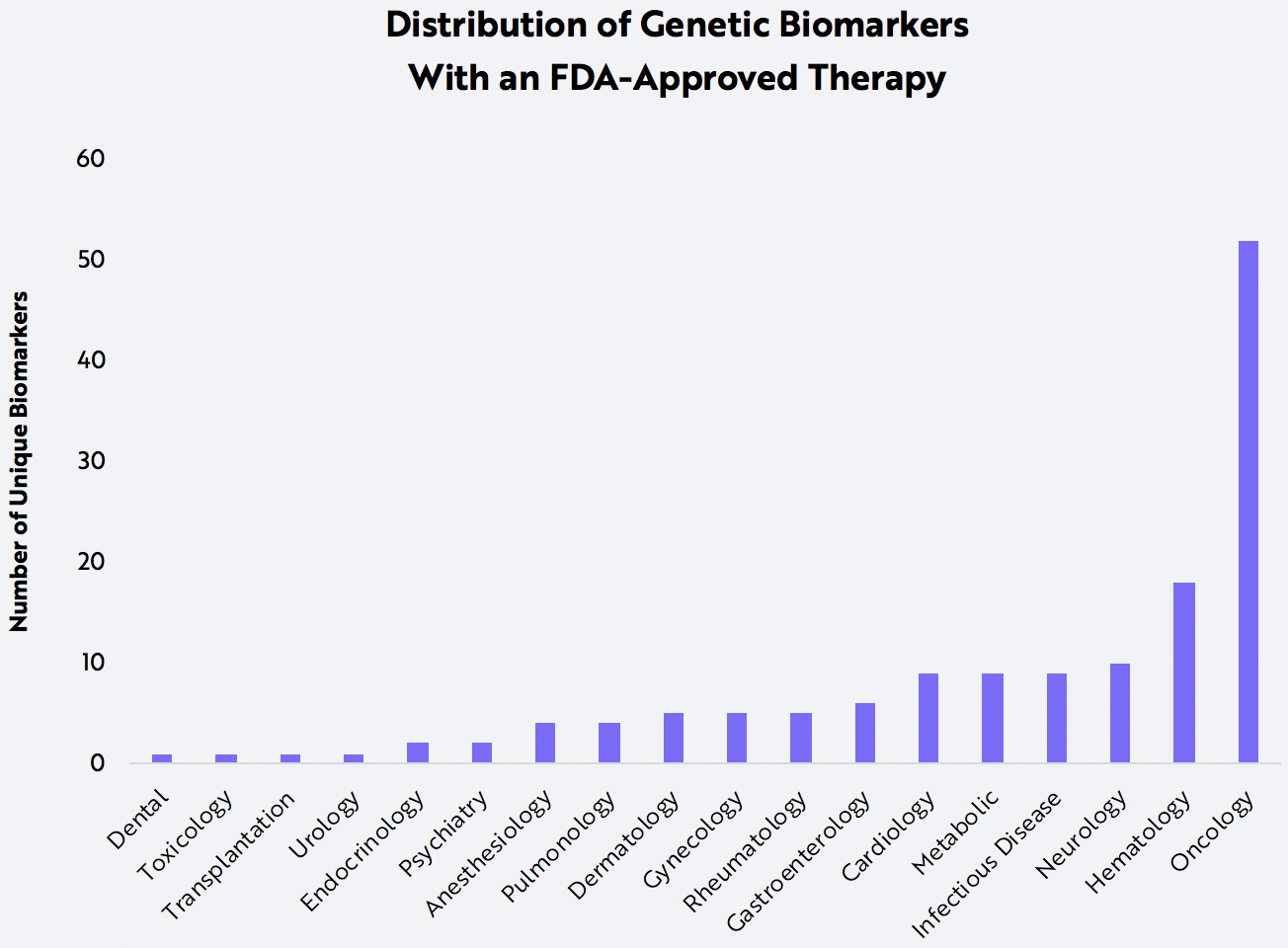
Source: ARK Investment Management LLC, 2020; Data Source: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling
Some mutations are not inherited and can appear spontaneously, giving rise to aggressive cells that coalesce into tumors. In the case of spontaneous variants, cancer patients are matched to targeted therapies with a lock-and-key system. First, using molecular diagnostic tests, oncologists search for the mutation—the lock—that is driving tumor growth. Then, diagnostic vendors introduce the molecular information to a genomic biomarker database and search for the best treatment—the key. Importantly, as the data on clinical outcomes feeds back into the system, the accuracy of the algorithms that match patients to therapies increases continuously.
The combination of NGS and therapy-matching also seems to be benefiting therapeutics companies working on targeted treatments. By either partnering with diagnostic vendors or bringing sequencing capabilities in-house, drug makers leverage NGS to find patients and enroll them in clinical trials. Genomic biomarker data offers therapeutics firms an information advantage that can translate not only into more capital-efficient clinical trial designs, but also into shorter trials and lower drug failure rates. Thanks to these efficiencies, trials that include genomic biomarkers are capturing share, as shown below.
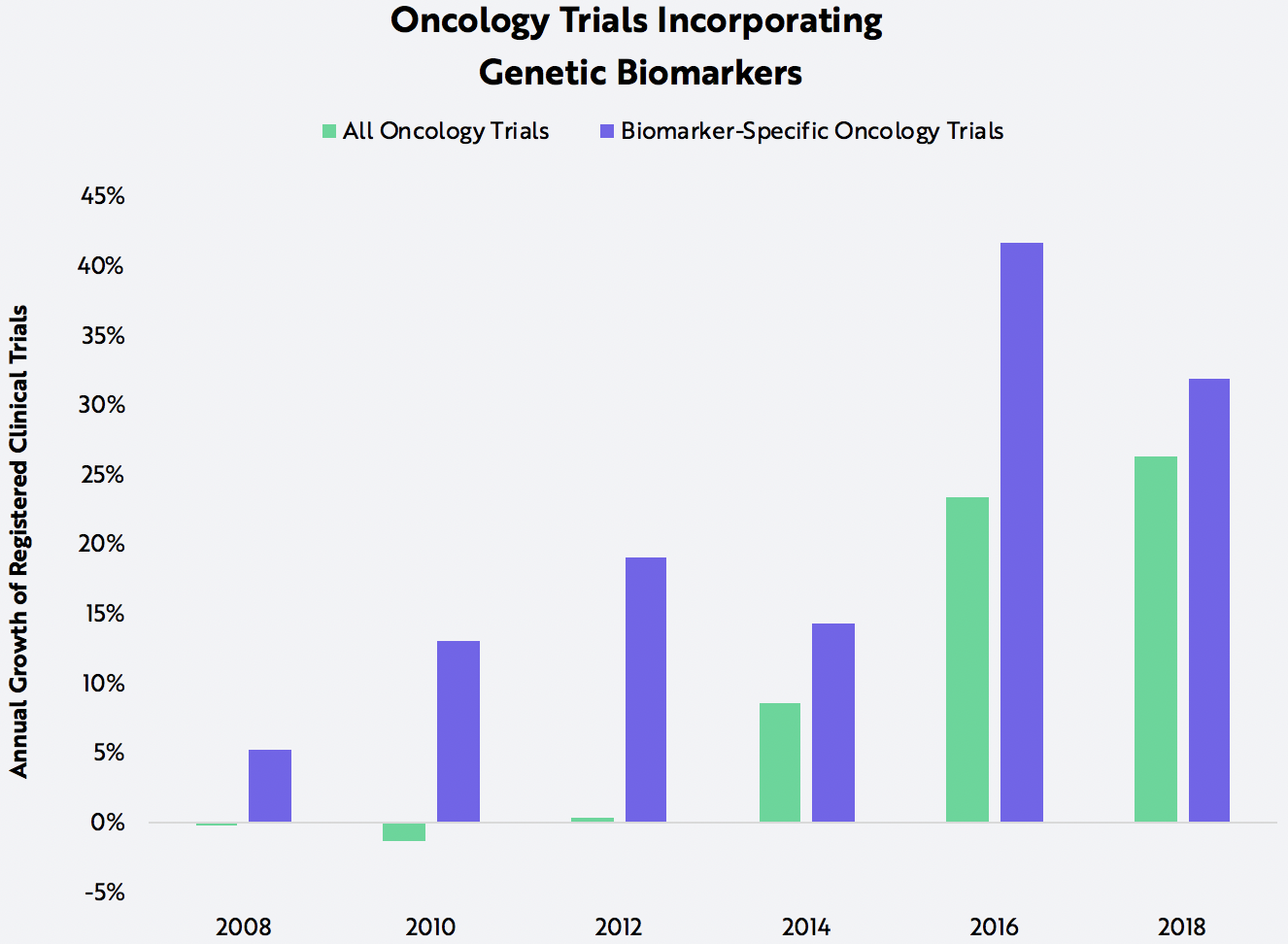
Source: ARK Investment Management LLC, 2020; Data Source: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling
In ARK’s view, the convergence of innovation platforms—NGS and artificial intelligence—is the forcing function that will turbocharge genetic medicine and transform personalized medicine from a dream into reality.As our understanding of tumor biology increases, ARK believes that the list of genomic biomarkers should grow dramatically. Soon, the majority of oncology trials may include genomic biomarkers, causing a boom in the number of targeted therapeutics. Lung cancer trials seem to be leading the way, as shown below. In turn, targeted therapies should cause NGS diagnostic panels to become more actionable, increasing both their cost-efficiency and the probability that public and private payers will reimburse them. They also should be a boon to algorithms that match biomarkers to therapies, increasing their effectiveness and mitigating their side effects. In our view, the convergence of innovation platforms—NGS and artificial intelligence—is the forcing function that will turbocharge genetic medicine and transform personalized medicine from a dream into reality.
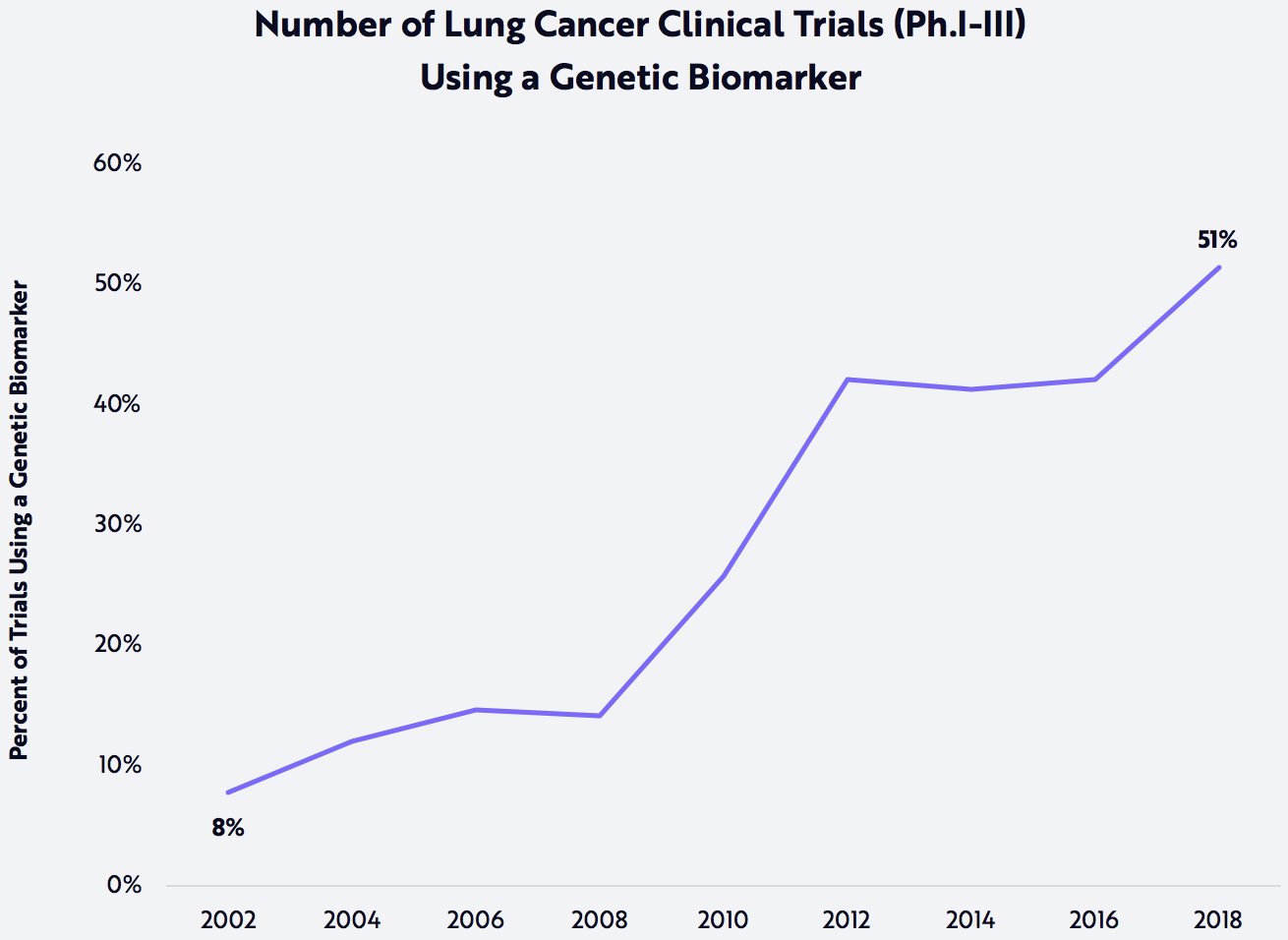
Note: Markers Include Tier-1 and Tier-2 Somatic Variants Associated with All Lung Cancer Indications in the COSMIC Database
Source: ARK Investment Management, LLC; Data Source: https://cancer.sanger.ac.uk/census


